
Please choose your preferred region

Echoport ILO292-II
Brochures
Manuals
The latest Echoport installation and software manuals are available below. Previous issues and translations are provided in the Support area.
Technical specifications
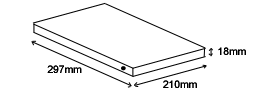
297mm x 210mm x 18mm (11.75in x 8.25in x 0.75in)
1.135kgm (2.6lbs)
Temperature: -40C to +70C (-40F to +158F)
Relative Humidity: 10% to 100%
Atmospheric Pressure: No requirements
Note: Unit must not be exposed to ingress of water or to severe physical trauma; degradation of unit function and/or performance may occur.
Note: Unit must not be exposed to ingress of water or to severe physical trauma; degradation of unit function and/or performance may occur.
Pentium II, 400 MHz, Windows 98 SE/ME/2000/XP, CD-ROM drive, USB port.
USB 1.1
Class II. The Echoport USB is powered from the computer to which it is connected.
For compliance with IEC601.1 any computer equipment connected to the USB interface of the Echoport USB must be certified to IEC standards (e.g. IEC 950) and configured to comply with IEC 601-1-1. Everybody who connects computer equipment to this interface configures a medical system and is therefore responsible for ensuring that the system complies with IEC 601-1-1. If in any doubt contact the technical service department of your local representative.
For compliance with IEC601.1 any computer equipment connected to the USB interface of the Echoport USB must be certified to IEC standards (e.g. IEC 950) and configured to comply with IEC 601-1-1. Everybody who connects computer equipment to this interface configures a medical system and is therefore responsible for ensuring that the system complies with IEC 601-1-1. If in any doubt contact the technical service department of your local representative.
Not protected against liquid ingress.
Portable equipment.
Not suitable for use with flammable gases.
In certain cases of extreme electromagnetic interference from external sources, the operation of the Echoport USB may be affected. The effects of any interference may take one of the following forms:
- The noise bar will periodically indicate a high level or the message ´TOO NOISY´ will be displayed. This behaviour will not correspond with acoustic/mechanical interference.
- The software may crash and report an error.
This OAE system consists of no user serviceable components. In the unlikely event of system failure, contact your local dealer who will arrange for your system to be serviced by an Otodynamics approved service engineer.
Warning : Removal of any access covers on the OAE system invalidates your warranty agreement.
ILO V6 Software
ILO V6 clinical OAE software opens up new windows on the cochlea. Designed by the originators of OAE technology and based on 25 years of experience with OAEs, V6 software is an expanding suite of carefully engineered OAE investigational facilities with proven clinical or scientific value. It also incorporates advanced data management services.
General features of ILO V6
- User friendly clinical/research software with integrated patient database
- Full range of advanced tests - TEOAE, DPOAE, SOAE, DP Growth, Binaural TE and DP, Contralateral Suppression
- Detailed Spectrum and Waveform analysis
- Flexible printing options including powerful examine and compare functions
- Password-protected user-defined protocols
- Easy to use probe calibration test with pre/post test comparison
- Exportable data to OZ, Hi Track, XML, ILOV5, Excel and ASCII
- Ability for distributed deployment across a network
- Compatible with previous ILO data
TEOAE recording
- Quickscreen and standard ILO nonlinear recording
- Tone pulse stimuli
- Adjustable stimulus intensity
- Real-time stimulus monitoring in Checkfit and during test
DPOAE recording
- DPgram with preset and custom selected stimuli
- Programmable level, level ratio, frequency ratio, points/octave, frequency range, manual/automatic/intelligent frequency progression
- Total DP power assessment
- DP growth rate analysis
- True time domain averaging and noise statistics
- Signal-to-noise assessment against two standard deviations from mean noise level
Advanced functions
- Powerful ′synchronised′ spontaneous emission search to reveal latent SOAEs
- DP Growth analysis
- Binaural TEOAE and DPOAE measurements
- Contralateral TEOAE suppression analysis
Analysis tools
- TEOAE, full cross correlation, frequency analysis with reproducibility and signal-to-noise data
- Examine/compare feature for TE-TE and TE-DP
- Response subtraction analysis
- Half-octave OAE power assessment
- Detailed spectrum analysis
- Full data table provided
- User configured ASCII output
- Waveform analysis
EZ-Screen Software
The EZ-Screen module has been specially designed to fulfil the needs of screening programmes. It is simple to use, intelligent to OAE responses and has highly automated test programmability.
Main features of EZ-SCREEN
- Easy to use TEOAE and DPOAE screening software with integrated patient database
- Flexible printing options including powerful examine and compare functions
- Password-protected user-defined protocols
- Easy to use probe calibration test with pre/post test comparison
- Integration of multiple sites, testers and machines into one database
- Exportable data to OZ, Hi Track, XML, ILOV5, Excel and ASCII
- Ability for distributed deployment across a network
- Compatible with previous ILO data

Otodynamics Ltd
30-38 Beaconsfield Rd
Hatfield, Herts
AL10 8BB, UK
30-38 Beaconsfield Rd
Hatfield, Herts
AL10 8BB, UK
UK Head Office: +44 1707 267540
USA Office: 1 800 659 7776
Fax: +44 1707 262327
USA Office: 1 800 659 7776
Fax: +44 1707 262327
sales@otodynamics.com
support@otodynamics.com
support@otodynamics.com
Registered in England
Company No: 2289571
VAT No: GB 539 9876 66
FDA Regn: 8021990
Producer Reg No: WEE/BF0358QU
 In the USA? click here
In the USA? click here




